millikan oil drop experiment results|August, 1913: Robert Millikan Reports His Oil Drop Results : Baguio The experiment was performed by spraying a mist of oil droplets into a chamber above the metal plates. The choice of oil was important because most oils would evaporate under .
Not only will you find any type of Escort Split, close to where you are located or even in Croatia, where you will be traveling in the near future, this place will also offer you the choice of selecting your favorite Escort, for endless and unlimited sexual pleasures.
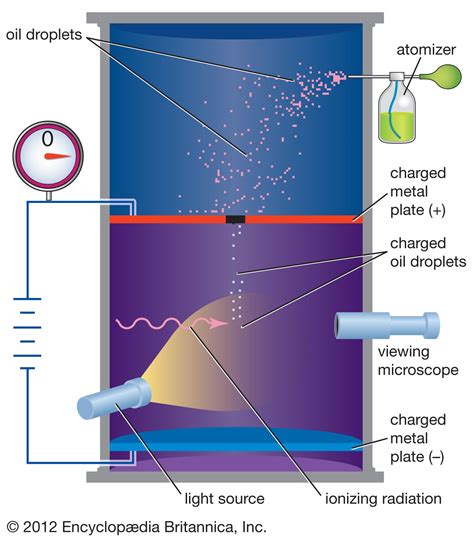
millikan oil drop experiment results,Millikan oil-drop experiment, first direct and compelling measurement of the electric charge of a single electron. It was performed originally in 1909 by the American physicist Robert A. Millikan, who devised a method of measuring the minute electric .charge conservation, in physics, constancy of the total electric charge in the .
The Millikens Oil Drop Experiment was an experiment performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the charge of .Robert Millikan’s famous oil drop experiment, reported in August 1913, elegantly measured the fundamental unit of electric charge. The experiment, a great .In a commencement address given at the California Institute of Technology (Caltech) in 1974 (and reprinted in Surely You're Joking, Mr. Feynman! in 1985 as well as in The Pleasure of Finding Things Out in 1999), physicist Richard Feynman noted: We have learned a lot from experience about how to handle some of the way.
August, 1913: Robert Millikan Reports His Oil Drop ResultsThe experiment was performed by spraying a mist of oil droplets into a chamber above the metal plates. The choice of oil was important because most oils would evaporate under .Millikan carried out a series of experiments between 1908 and 1917 that allowed him to determine the charge of a single electron, famously known as the oil drop experiment. .Millikan repeated the experiment for over 150 oil drops. He selected 58 of his results and found the highest common factor. That is, the single unit of charge which could be multiplied up to give the charge he measured on .

As in Millikan’s original experiment, oil drops are sprayed into a region where a uniform electric field can be established, and the motions of drops are studied under the action .
millikan oil drop experiment resultsIn an important series of experiments in Chicago, Robert Millikan measured the charge on a series of oil drops. He did this by observing the time taken for a drop to fall at its .Oil drop experiment. Robert A. Millikan.. (1909). q=1.5924(17)×10−19 C. Shot noise experiment. First proposed by Walter H. Schottky. In terms of the Avogadro constant . Regardless, it is Millikan whose results have stood the test of time, and whose name remains associated with oil drops and electrons in physics textbooks today. . My work with Millikan on the .millikan oil drop experiment results August, 1913: Robert Millikan Reports His Oil Drop Results Regardless, it is Millikan whose results have stood the test of time, and whose name remains associated with oil drops and electrons in physics textbooks today. . My work with Millikan on the .Balance of Forces: Newton’s Law a : radius of drop ρ: density ρ= ρ oil –ρ air v: velocity of oil drop Q: charge of oil drop E: electric field E=V/d V : Voltage across plates η: viscosity of air g : gravitational const. Ö ()) 6 1) dr g dr a ag E g E Fa gz FQ dv F v t E d Ö zg 6 vrag QEE Forces on the oil drop:
This chapter is devoted to Millikan's oil drop experiment. After a brief historical introduction, we describe the experiment in detail, with a careful treatment of the physics involved. . The results of Michael Faraday's experiments on electrolysis started to put forward the idea that electric charge may come in discrete units, in some way .
Robert Millikan's oil drop experiment measured the charge of the electron. The experiment was performed by spraying a mist of oil droplets into a chamber above the metal plates. The choice of oil was important because most oils would evaporate under the heat of the light source, causing the drop to change mass throughout the experiment. .– Millikan's oil drop experiment (ACSPH026) . Subsequently, Millikan calculated the charge for numerous droplets, each different in mass (enhanced reliability of results) Results – Millikan that the charges of differently sized droplets were quantised in the sense that they are multiples of a certain number.
The Millikan oil drop experiment, published in final form in 1913, demonstrated that charge comes in discrete chunks and was a bridge between classical electromagnetism and modern quantum physics. . But Felix Ehrenhaft of the University of Vienna repeatedly challenged Millikan’s results, based on his own measurements of .By adjusting the electric field strength and making careful measurements and appropriate calculations, Millikan was able to determine the charge on individual drops (Figure \(\PageIndex{2}\)). Figure \(\PageIndex{3}\): Millikan’s experiment measured the charge of individual oil drops. The tabulated data are examples of a few possible values.
The Millikan oil drop experiment, published in final form in 1913, demonstrated that charge comes in discrete chunks and was a bridge between classical electromagnetism and modern quantum physics. . But Felix Ehrenhaft of the University of Vienna repeatedly challenged Millikan’s results, based on his own measurements of .
Background In Millikan’s experiment, oil drops are sprayed from a nozzle into a volume between two closely-spaced horizontal metal plates that are insulated from each other. The drops can be kept from falling by . Millikan's Oil Drop Experiment 1. Were your results on the number of electrons always integers?Balance of Forces: Newton’s Law a : radius of drop ρ: density ρ= ρ oil –ρ air v: velocity of oil drop Q: charge of oil drop E: electric field E=V/d V : Voltage across plates η: viscosity of air g : gravitational const. Ö ()) 6 1) dr g dr a ag E g E Fa gz FQ dv F v t E d SK Ö zg 6SK vrag QEE Forces on the oil drop:
The success of the Millikan Oil-Drop experiment depends on the ability to measure small forces. The behavior of small charged droplets of oil, weighing only 10−12 gram or less, is observed in a gravitational and electric field. Measuring the velocity of fall of the drop in air enables, with the use of Stokes’ Law, the calculation of the .
The Oil Drop Experiment was performed by the American physicist Robert A Millikan in 1909 to measure the electric charge carried by an electron. Their original experiment, or any modifications thereof .
Millikan repeated the same experiment thoroughly for over 150 oil drops and selected 58 of Millikan oil drop experiment results and got to find the highest common factor. It means the single unit of charge that could be multiplied up to give the charge he measured on all of his oil drops. Oil Drop ExperimentMillikan Oil Drop Apparatus Introduction 2 012-13093C where m is the mass and g is the acceleration due to gravity. To eliminate m from equation ( 3 ), the expression for the volume of a sphere and the density of the oil are used: where a is the radius of the droplet, and is the density of the oil. Substituting equation ( 4 ) into equation ( 3 ) yields:

"Robert A. Millikan," by Daniel J. Kevles, Scientific American, 240, pp 142-151 (January 1979). "My Work With Millikan On the Oil-drop Experiment," by Harvey Fletcher, Physics Today, pp 43-47 (June 1982). For a detailed analysis of the Millikan's work on the oil-drop experiment, including what he wrote in his laboratory notebooks see:The purpose of Robert Millikan and Harvey Fletcher's oil-drop experiment (1909) was to measure the electric charge of the electron.They did this by carefully balancing the gravitational and electric forces on tiny charged droplets of oil suspended between two metal electrodes.Knowing the electric field, the charge on the oil droplet could be .
Millikan’s setup was based on the equilibrium of forces of small charged particles (oil bubbles in this case) in an electric field (Fig. 1.) ++ ++++ ++++ +++-----+ Fig. 1: Millikan Setup If the experiment is performed under normal conditions (e.g. in air), four major forces act on the particle. Coulomb Force and buoyancy are pushing the
case study of Millikan s oil drop experiment Sapna Sharma and P K Ahluwalia-This content was downloaded from IP address 207.46.13.2 on 01/07/2022 at 23:10. IOP PUBLISHING EUROPEAN JOURNAL OF PHYSICS . experiment. As a result, several suggestions were made as to how the effectiveness of the
millikan oil drop experiment results|August, 1913: Robert Millikan Reports His Oil Drop Results
PH0 · The Millikan experiment
PH1 · The Millikan Oil Drop Chemistry Experiment
PH2 · Oil drop experiment
PH3 · Millikens Oil Drop Experiment
PH4 · Millikan's Oil Drop Experiment
PH5 · Millikan oil drop experiment
PH6 · Millikan oil
PH7 · Millikan Oil Drop Experiment
PH8 · MILLIKAN OIL DROP EXPERIMENT
PH9 · August, 1913: Robert Millikan Reports His Oil Drop Results
PH10 · 4.12: Oil Drop Experiment